Today’s Managing Health Care Costs Indicator is 97%
This week’s Annals of Internal Medicine has two articles about NovoSeven, a bioengineered medication to replace the missing blood factor that leads hemophiliacs to have bleeding problems.
First of all – NovoSeven is a miracle drug. About a fifth of hemophiliacs develop antibodies against clotting factor concentrate. These hemophiliacs need progressively higher doses of factor concentrate, and sometimes continue to bleed anyway. NovoSeven. The drug takes a year to make, and most of that time is spent checking to be sure that it’s pure.
Of course, you won’t be surprised the drug is expensive! It costs $10,000 a dose – largely because it is priced as an orphan drug. There are only 20,000 hemophiliacs in the US – so it’s a small market.
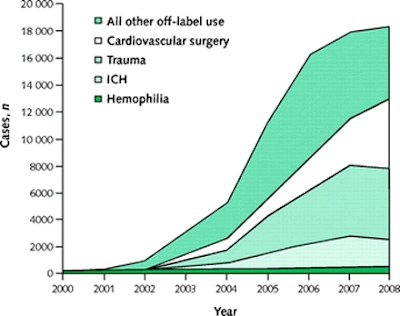
But not SO small. In 1999, a case report from the Israeli military showed that NovoSeven could arrest life-threatening bleeding from trauma. Since then, the drug has been used off-label for brain surgery in those that are on anticoagulants, as well as for trauma surgery and even coronary bypass surgery (CABG). In fact, 97% of the NovoSeven used in an inpatient setting are for reasons other than hemophilia. It’s used off-label more outside of academic medical centers (but it’s more likely that hemophiliacs get cared for at major teaching hospitals).
This is not on the radar screen for most health plans, since this cost is bundled into DRG or per diem hospital payment.
The Agency for Health Research and Quality (AHRQ) funded this study, and a companion literature review that shows no benefit from clinical trials using NovoSeven for these indications, and some incremental blood clotting danger. Patients exanguinating in the operating room are more likely to make it back to the ICU – but not more likely to live to hospital discharge.
The off-label use of NovoSeven is why we need a robust investment in comparative effectiveness research. There’s no evidence that Novo Nordisk promotes this off-label use, and there are no greedy providers throwing in a high-margin drug. Everyone deeply believed that this drug which made bleeding vanish would save lives.
There was no reason for Novo Nordisk to study this – and the cost of NovoSeven for these off-label uses skyrocketed. The drug needed to be studied – and it made sense for government to sponsor this research. The “return on investment” for this comparative effectiveness research will be huge.
Here’s a link to a recent NEJM article showing positive ROI for comparative effectiveness research into cement for spinal surgery.