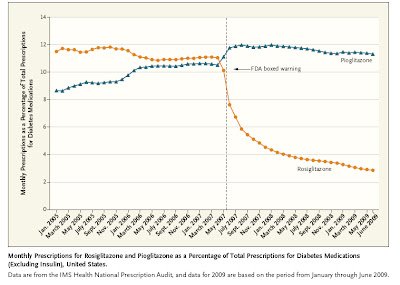
The initial study showing that this medicine appeared to be 'trouble' was a metaanalysis published in 2007, and accompanied by an FDA "black box" warning. The good news is that this new information and warning was associated with an impressive ~45% drop-off in drug utilization. We often worry about the slow pace of incorporation into practice of new information. In this instance the communication around the dangers of rosiglitazone looks like a big success.
click to enlarge
These images come from an article in the New England Journal of Medicine on November 25.
Here's a worry though. Look at the geographic variation of use of this drug from 2005 to 2010.
Click image to enlarge
The dropoff in rosiglitazone use is pretty uniform across the country- about 75% decrease in use for each quartile of previous utilization. (The authors don't give state-specific data). However, the northern plains and New England states had a low rate of use of this medicine in the first place -and maintained this over time. Use of this (expensive) medicine represented an exceptionally large portion of diabetes medication costs in states including Idaho, Utah, Wyoming, Oklahoma, and Kentucky in 2005. Although these states used the highest amount of an exceptionally expensive diabetes medicine - they are not states known for differentially higher quality diabetes care.
This is another illustration of the Dartmouth Atlas contention. Variations in overall cost usually don't purchase better quality - and sometimes purchase higher risk.
This is another illustration of the Dartmouth Atlas contention. Variations in overall cost usually don't purchase better quality - and sometimes purchase higher risk.